Kurzbeschreibung
The students transform chemically neutral water into carbonated water by adding carbon dioxide. The change in pH is measured with a universal indicator. Afterwards, the liquid is restored to neutral by heating it, which drives out the carbon dioxide again.
This resource is part of the educational kit "The Climate Box". You can read more about the kit in the presentation attached. Find all related resources selecting the category "Our fragile planet" and "secondary level".
Ziele
The students learn how water by solving carbon dioxide can change from a life supporting to a life threatening liquid. They will realise that the progressing release of CO2 not only drives the greenhouse effect but also affects the marine ecosystem through acidification. Oceans are a carbon sink that help mitigate the greenhouse effect, but experience disadvantageous consequences on their own. The students also learn that the amount of CO2 solved in water depends on its temperature.
Lernziele
The students will learn basic chemistry concepts e.g. that adding an agent to another can change the characteristics. Here, they learn that solving a gas like carbon dioxide in a life supporting liquid like water can change its character quite drastically. In this case, the result can even harm life as we know it. They learn how to determine that change and what the results mean in the global picture.
Bewertung
Activity 1
Before the activity
After introducing the topic as suggested in the description of the activity, the teacher can probe the current knowledge of the students by asking some questions (also mentioned in the description).
Question: What substances do you know that contain carbon?
Possible answers: coal, petrol, fuel, pencils, sugar, carbon dioxide, methane, …
Question: Do you know solid, liquid or gaseous substances that contain carbon?
Possible answers: (see above)
Question: What is simplest gas that contains carbon and that is a product of combustion or organic decay? You even exhale it when breathing.
Answer: carbon dioxide (CO2)
Question: What happens with carbon dioxide, when it is solved in water, as it happens with the oceans on Earth?
Possible answers: carbonated water, enriches water with carbonic acid
Question: What do acids do?
Possible answers: They can dissolve material.
During the activity
Question: What is the pH value of water? Is the liquid acidic or basic?
Answer: The pH value is 8, which means the water is neutral or slightly basic.
After the activity
Question: What is the pH value you reached? Did the water become acidic or basic?
Answer: The pH value is … (3 in the example given in Figure 12). This is quite acidic.
Question: What do acids do with other substances?
Answer: Acids have the power to dissolve certain substances.
Question: What happens to marine creatures with exoskeletons made of limestone, when the water becomes more acidic?
Possible answers: The limestone is dissolved by carbonic acid.
Activity 2
Before the activity
Question: Do you have an idea how to neutralise the solution, i.e. change the acid into neutral water again? The teacher can help by telling that the gas of sparkling water and sodas is carbon dioxide as well. What happens to them when keeping them open at room temperature for a while?
Possible answers: drive out the carbon dioxide by waiting for a long time or support by heating
During the activity
Question: Do you see a change while heating the solution?
Answer: Yes, the colour changes.
After the activity
Question: What is the pH value you reached?
Answer: The pH is back to a neutral value of 7 to 8.
Question: What happens to ocean water when it is transported from polar to equatorial regions?
Answers: It heats up.
Question: Which waters can store more carbon dioxide? Polar or equatorial?
Answers: polar
Question: So, what happens to the carbon dioxide solved in the ocean?
Answer: It is released into the atmosphere.
Materialien
The number of items corresponds to the number of students carrying out the experiments. One set consists of:
- Distilled or demineralised water
- Transparent cup or glass
- Straw
- Universal pH indicator according to McCrumb (http://pubs.acs.org/doi/abs/10.1021/ac50075a004), see also: http://www.hometrainingtools.com/universal-indicator-30-ml and http://experilab.co.za/catalog/index.php?main_page=product_info&products_id=602
- Small heater or stove
The heater is only needed for the second part of the activity, where the water is neutralised. For safety reasons, the teacher may decide to carry out this experiment as a demonstration only.
Hintergrundinformationen
The carbon cycle
The Earth is a dynamic system that exchanges energy and materials between different spheres and outer space. One of the important circulation systems is the carbon cycle.

Figure 1: The annual flux of CO2 in GigaTons (Gt) or billions of tons between each of the Earth's reservoirs. Each reservoir serves as both a source of and a sink for carbon, as indicated by opposing arrows. The carbon released by burning fossil fuels is an unbalanced contribution to the global carbon budget. The total contribution of carbon from the burning of fossil fuels has increased from 5.5 Gt to between 7 and 8 Gt from 2003 until 2007 (Credit: NASA/AIRS, https://www.flickr.com/photos/atmospheric-infrared-sounder/8265010034, https://creativecommons.org/licenses/by/2.0/legalcode).
Carbon is altered chemically and its compounds attain different physical states. Usually, the exchange of carbon between the lithosphere, the hydrosphere, the biosphere, and the atmosphere is maintained in a delicate and naturally balanced equilibrium, with carbon sources and carbon sinks being in constant interaction. Sinks and sources are defined as subsystems that capture carbon or release it into the atmosphere where they act as greenhouse gases like carbon dioxide or methane.
Table 1: Natural and artificial carbon sources and sinks.
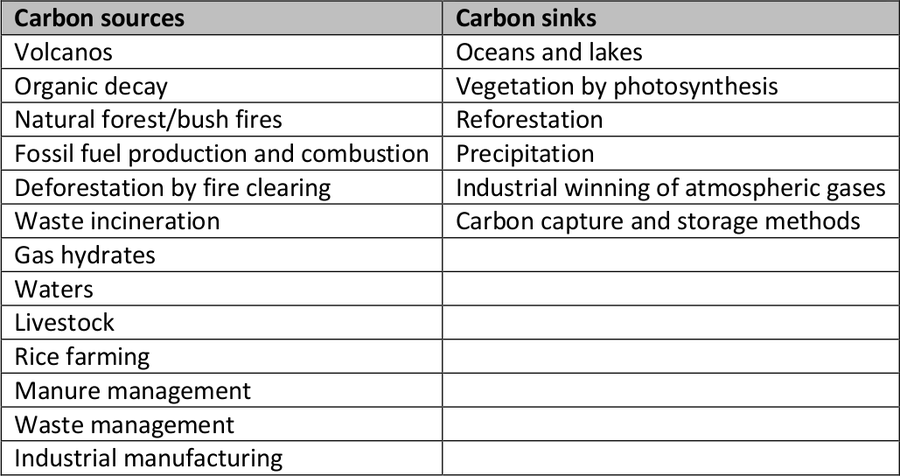
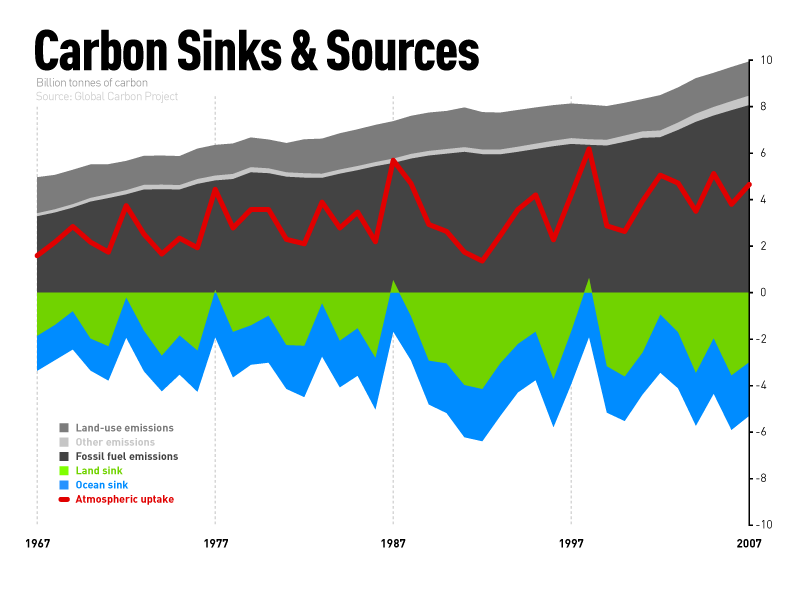
Figure 2: Evolution of the budget of carbon sinks and sources (Credit: climatesafety, https://www.flickr.com/photos/climatesafety/4745854611, https://creativecommons.org/licenses/by-nc/2.0/legalcode).
However, artificial interference by human activities constantly increases a net imbalance towards carbon sources leading to a growing concentration of carbon based greenhouse gases. As Figure 3 illustrates, the amount of atmospheric CO2 has increased dramatically since the beginning of the 20th century. The growth rate is unprecedented for the recent several hundred thousand years. There is a broad consensus among climatologists that this contributes significantly to the global warming we measure today. Carbon dioxide concentrations can be measured both by sensors on ground and with dedicated Earth observation probes from space by remote sensing. Successful space programmes for monitoring greenhouse gases globally are Europe’s Envisat, Japan’s GoSat as well as NASA’s OCO-2 satellite. Europe’s Copernicus programme with its Sentinel satellites will help understand the effects of an increasing level of greenhouse gases released into the atmosphere.
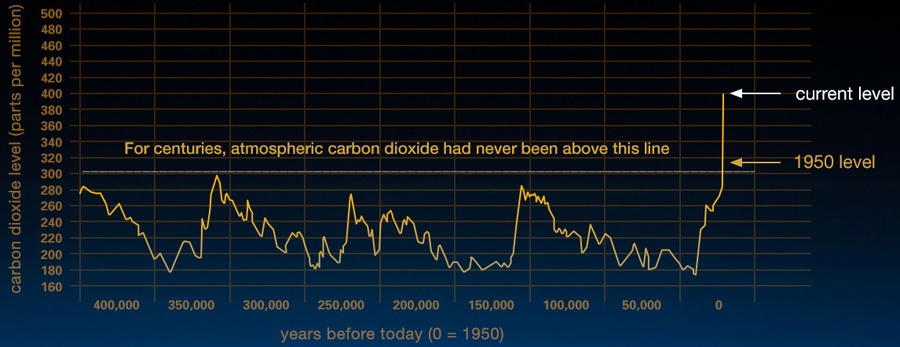
Figure 3: This graph, based on the comparison of atmospheric samples contained in ice cores and more recent direct measurements, provides evidence that atmospheric CO2 has increased since the Industrial Revolution until February 2016. (Credit: Vostok ice core data/J.R. Petit et al.; NOAA Mauna Loa CO2 record/NASA/JPL, http://climate.nasa.gov/evidence/, public domain).
The pH value
The pH value is a measure for the strength of acids. Its value represents the concentration of free hydron (H+) or hydronium (H3O+) ions. The value is defined as:
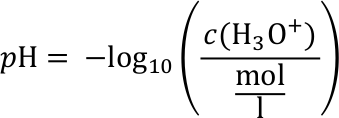
The concentration of hydronium ions c(H3O+) is given in units of moles per litre. The mole is a standard unit for the amount of a given substance. There are indicators that change their colour depending on the pH value of the solution. This permits measuring the pH value.
The oceans as a carbon sink
Up to 30-40% of the manmade carbon dioxide is captured in oceans, rivers and lakes. The gas is efficiently solved in water. Therefore, oceans are a very powerful and significant carbon sink.
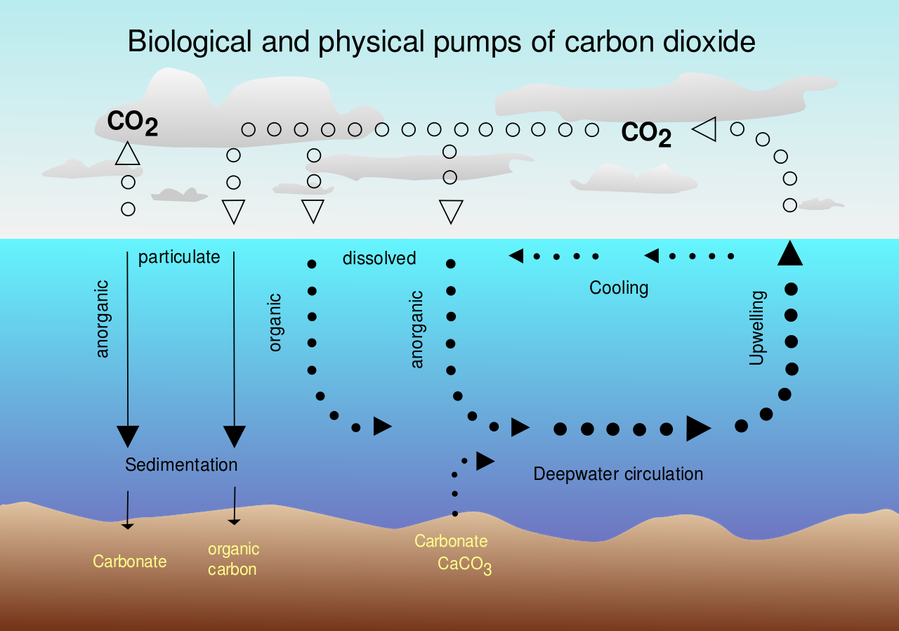
Figure 4: Air-sea exchange of carbon dioxide (Credit: McSush (modified), Hannes Grobe (original), https://commons.wikimedia.org/wiki/File:CO2_pump_hg.svg, https://creativecommons.org/licenses/by-sa/2.5/legalcode).
Although the ability of water capturing and storing CO2 helps reducing greenhouse gases, it comes with a high price. Solving CO2 in water changes the chemistry. As a result, the water becomes more acidic. The acidification and its consequences can be split up into three chemical reactions. First, carbon dioxide and water form carbonic acid.
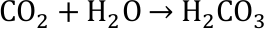
The acid is immediately split up into its ions, among which is the hydron ion which also reacts to form the hydronium ion H3O+. The free hydron or hydronium ions are characteristic for an acid. This is reflected in the definition of the pH value (see above).
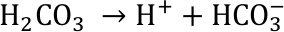
The acidic solution reacts with carbonate ions that are abundant in ocean water. They are the building blocks e.g. for the exoskeletons of shellfish like snails, mussels as well as corals.
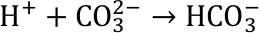
Those reactions occur at the surface of waters like the oceans. As a result, the formation of carbonate compounds like lime is hindered, or in extreme cases, existing exoskeletons can even be dissolved. The net equation of the reaction chain is shown in Figure 5.
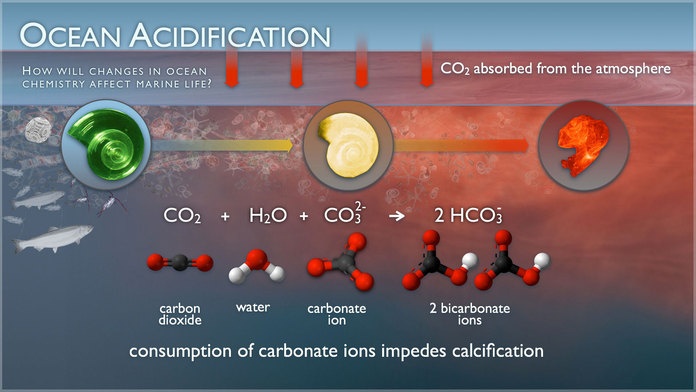
Figure 5: Illustration of how solving CO2 in water consumes carbonate ions. It impedes calcification or even may lead to decalcification of sea shells (Credit: NOAA PMEL Carbon program, public domain).
Although the degree of salinity of sea water mitigates the effect of acidification, the tendency still remains. Apart from in situ sample measurements, new technologies exist to determine the ocean pH levels on a global scale using remote sensing from Earth observation satellites (Figure 6).
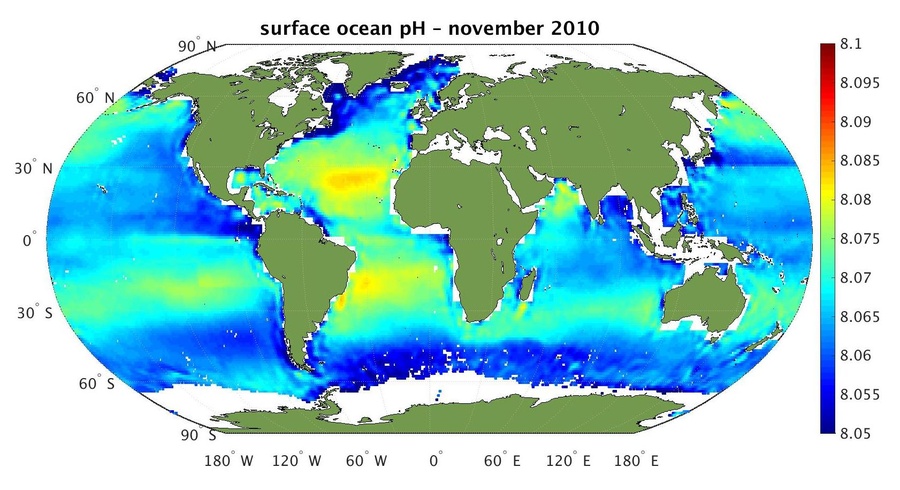
Figure 6: This map shows the first estimates of surface ocean pH using salinity data from ESA’s SMOS with satellite sea-surface temperature measurements and additional auxiliary data. There is a spatial variation of the pH across the globe. Cold waters near the poles tend to be more acidic due to the ability of cold water to solve carbon dioxide better than warm water (Credit: ESA/R. Sabia, http://www.esa.int/spaceinimages/Images/2015/01/Surface_ocean_pH, https://creativecommons.org/licenses/by-sa/3.0/igo/legalcode).
Such maps also indicate that especially polar regions are stronger affected by acidification than others. This can be explained by the phenomenon of cold water being able to solve CO2 better than warm water. Wide range water currents are known to connect the oceans of the world. As a consequence water is exchanged between latitudes, and so acidic, i.e. CO2 rich, water is transported from the poles to the equator regions. The water is heated up on its way and releases part of the stored CO2. Therefore, oceans can also be regarded as a regionally confined carbon source.
This influence of water temperatures is also confirmed by data models that result in a historic and projected evolution of global pH levels as shown in climate reports of the IPCC (Intergovernmental Panel on Climate Change, see Figure 7). All projections result in a stronger acidification of the polar regions as compared other regions on Earth.
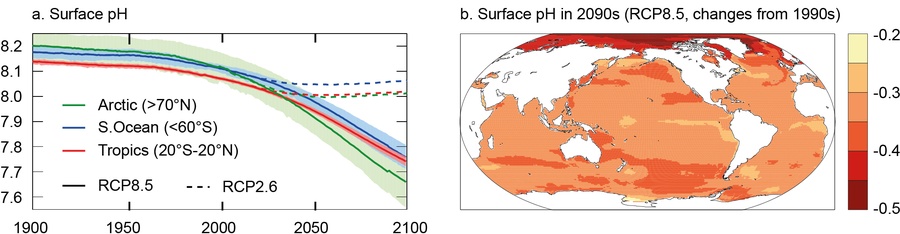
Figure 7: Historic and projected evolution of oceanic surface pH levels. The models were calculated for the most optimistic (RCP2.6, Representative Concentration Pathways) and the most pessimistic scenarios (RCP8.5) for the evolution of atmospheric CO2. (a) Time series of surface pH shown as the mean (solid line) and range of models (filled), given as area-weighted averages over the Arctic Ocean (green), the tropical oceans (red) and the Southern Ocean (blue). (b) Map of the median model’s change in surface pH from 1990 to 2090 (Credit: IPCC Report, 2013, Working Group I, Chp. 6, p. 532, permission for reproduction granted).
Vollständige Beschreibung der Aktivität
Introduction
Introduce the topic by telling the pupils that carbon is the main element in living things. Each compound contains carbon atoms.
Question: What substances do you know that contain carbon?
Question: Do you know solid, liquid or gaseous substances that contain carbon?
Question: What is simplest gas that contains carbon and that is a product of combustion or organic decay? You even exhale it when breathing.
The atmosphere of the Earth contains carbon dioxide whose abundance is constantly rising.
Question: What happens with carbon dioxide, when it is solved in water, as it happens with the oceans on Earth?
This is what we will find out with the next activity. It turns into an acid.
Question: What do acids do?
Information:
The strength of an acid is measured with the pH value. The scale runs from 1 to 14. A neutral substance that is neither an acid nor lye has values close to 7. The lower the value, the stronger the acid is. High values indicate lye. The pH value can be measured with indicators that change colour accordingly.
Activity 1
List of Material
The number of items corresponds to the number of students carrying out the experiments. One set consists of:
- Distilled or demineralised water
- Transparent cup or glass
- Straw
- Universal pH indicator according to McCrumb (http://pubs.acs.org/doi/abs/10.1021/ac50075a004)
Hand out the items to the students. Let them perform the following tasks step by step. Warn them not to swallow the liquid.
Step 1: Pour some water in the glass.

Figure 8: Items required for the first part of the activity (own work).
Step 2: Add a few drops of the indicator until the solution becomes green.
Question: What is the pH value? Is the liquid acidic or basic?

Figure 9: The colour of the water after adding some pH indicator is consistent with a pH value of 8 (own work).
Step 3: Use the straw und blow gently into the solution. Take care that it does not spill out of the glass. Continue until the colour of the indicator does not change any longer.

Figure 10: Blowing air into the liquid adds carbon dioxide, which slowly changes the pH value (own work).
Step 4: Compare the final colour with the pH table of the indicator and determine the pH value.

Figure 11: After blowing for several minutes, the colour stops changing. In this example, a pH value of 3 was reached. It indicates a change to acidic carbonated water (own work).
Question: What is the pH value you reached? Did the water become acidic or basic?
Question: What do acids do with other substances?
Information:
In this case, the students produced carbonic acid. In nature, the products react with limestone, which chemically is calcium carbonate. It is the building block of the exoskeletons of many marine creatures like sea snails, mussels or corals.
Question: What happens to marine creatures with exoskeletons made of limestone, when the water becomes more acidic?
Activity 2
Ask the students, if they have an idea how to undo Activity 1, i.e. how to neutralise the solution again. Tell them that sparkling mineral water and sodas also contain solved carbon dioxide.
Ask them what happens to the soda, if it is kept at room temperature for a while.
The following experiment can be carried out as a demonstration by the teacher, if there is the possibility that the students might hurt themselves when heating the solution. Otherwise, hand out heaters or stoves and turn them on.
Put the glass with the acidic solution on the heater. After heating it for several minutes, the liquid starts to change colours. Avoid boiling! Eventually, the colour should turn to green indicating neutralisation. The different shades of the colours are shown in Figure 12.

Figure 12: When heating the acidic liquid, the colour starts to change again. The acidification is reversed, because the carbon dioxide is driven out of the liquid. Warm water cannot store as much carbon dioxide as water at lower temperatures (own work).
Question: What is the pH value you reached?
Question: What happens to ocean water when it is transported from polar to equatorial regions?
Question: Which waters can store more carbon dioxide? Polar or equatorial?
Question: So, what happens to the carbon dioxide solved in the ocean?
Lehrplan
Space Awareness curricula topics (EU and South Africa)
Our fragile planet, Oceans, biodiversity
Fazit
The pupils turn neutral water into a carbonic acid by adding carbon dioxide when blowing into the solution. Afterwards, they neutralise the solution by heating it which releases the solved carbon dioxide again. This experiment is an analogy of what happens to water when exposed to the main greenhouse gas, carbon dioxide, i.e. it solves the gas and becomes more acidic. This is a threat to marine species like corals and snails who build exoskeletons by building limestone structures. Limestone is dissolved by carbonic acid. In the end, the students see that warm water can store less carbon dioxide which in turn is less acidic. However, ocean water is transported from polar to low latitude regions. On its way, it releases carbon dioxide that cannot be stored due to the heated water.

This resource was developed by Markus Nielbock, Haus der Astronomie, Heidelberg, Germany. This resource is under peer-review, proof reading, and will be updated and improved in the coming year.

